Ammonia is a colorless gas that exists in trace amounts in our atmosphere. It is a “sticky,” highly reactive form of nitrogen that can be produced industrially (for agricultural or military uses, for example) or naturally. When proteins degrade or are metabolized, one of the primary products is ammonia.
Because ammonia is considered a “reactive nitrogen species” (RNS), it plays a role in many, many ecological processes. It dissolves readily into surface water – lakes, streams, even dew – and in atmospheric water like clouds and fog. From there it makes its way into all sorts of chemical and biological nitrogen pools, either being re-assembled into amino acids and proteins, or being transformed into other inorganic nitrogen species like nitrate and nitrite. While it is in the atmosphere, it can react with acidic gases like nitric oxides and sulfur dioxide to form extremely small particles that travel great distances in air masses.
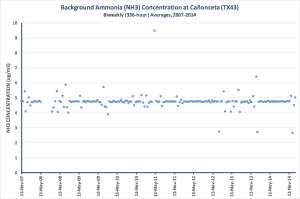
We’ve been monitoring background ammonia concentrations at Cañonceta since 2007. The overall trend at that site is an ever-so-slight, almost imperceptible decrease. The long-term average (~7 years) concentration is just shy of 5 MICROgrams per cubic meter.
“So what?” you ask. It’s not much to worry about. Most people can’t even smell ammonia at that level. Health effects? Unlikely. Ammonia’s regulatory thresholds for occupational health are in the range of 18,000 to 35,000 MICROgrams per cubic meter. That’s 3,600 to 7,000 times as high as we’re measuring.