Proc. 57th Southern Pasture and Forage Crop Improvement Conference, Athens, GA April 23-25, 2002
Sampling and Testing for Endophyte Technology of Tall Fescue.
Nick Hill, Dept. Crop and Soil Sciences, University of Georgia.
Recent developments utilizing non-toxic isolates of Neotyphodium spp. for forage improvement has changed thinking on endophyte technology and the necessity of sampling and testing for quality control purposes. Historically, there has been a negative connotation with endophytes, but now their role for developing value-added non-toxic forages have been recognized through manipulation of plant/endophyte mutualistic association. Thus, as a scientific group we need to be aware of concerns as to how accurate are the testing procedures, how rigorous testing protocols might be, and how much faith we can place in the testing methods. These concerns are valid whether testing within our academic disciplines (plant breeding programs, plant/endophyte physiology studies, animal grazing studies), or in regulatory agencies (seed labeling, etc.). The rigors of testing are dependent upon the testing venue (academic vs. regulatory vs. outreach) AND/OR the type of academic excercise undertaken. This paper will deal with some of the issues, concepts, and conclusions developed based upon extensive endophyte and toxicity testing in scientific and commercial settings.
There are two basic methods in which endophytes can be detected. Histological staining and examination of tissue under a microscope was the first standard method developed. Microscopic examination requires that a skilled technician observe the classical circuitous endophytic mycelial characteristic when growing between cells of stem sheath tissue or within aluerone layers of the seed (Welty et al., 1986a). This method was embraced by regulatory agencies when recommendations for use of endophyte-free seed prevailed (Welty et al., 1986b). Immunochemical methods have also been developed for use in ELISA or immunoblot methods (Welty et al., 1986; Reddick and Collins, 1988; Gwinn et al., 1991; Hiatt et al., 1997). The ELISA methods are sensitive, specific, consistent and capable of performing analyses on large numbers of samples, but require expensive laboratory equipment and extensive preparation to perform the analysis. Tissue-print immunoblot techniques were first proposed by Gwinn et al. (1991) using polyclonal antibodies. The technique worked equally well on seed and tiller tissue, but seed analysis was erratic unless scarified, imbibed in water, and split in half. The method has since been simplified and intact seed can be analyzed for endophyte (Hiatt et al., 1999). The method has been approved by the International Seed Testing Association for use in tall fescue and perennial ryegrass (Hill et al., 2002). A word of warning: antibodies used for immunochemical detection can be produced in a variety of ways and not all have equal specificity to the target entity. Polyclonal antibodies are found in the serum, and are composed of an almost infinite array of molecules of varying affinities and quantities. Over 90% of antibodies extracted from whole serum have no specificity to the target antigen (Roitt, 1994). Hence, there is a propensity for false positives using polyclonal formats. Immunochemical systems based upon monoclonal antibodies are more specific because they isolate and culture cells producing the antibodies. The antibodies are screened for their specificity to the target antigen and, thus, antibodies which have no cross reaction with taxonomically related organisms are less likely to give false readings (Goding et al., 1986).
Use of non-toxic endophytes in tall fescue breeding necessitates testing for ergot alkaloids as well as endophyte presence. Initial studies using HPLC for alkaloid analysis in breeding and genetics studies proved costly, time consuming (Hill et al., 1993; Agee et al., 1994; Roylance et al., 1994) and ill-suited for the extensive testing that a breeding or genetics program entails. Antibodies were developed to the lysergic moiety of the ergot alkaloids and an ELISA system developed for quantitative and/or qualitative alkaloid analysis (Hill et al., 1994; Hill and Agee, 1994). Therefore, an efficient double-screening system (endophyte and toxins) has been developed by which plant breeders, geneticists and other forage scientists can reliably and efficiently test for endophytes and ergot alkaloids.
Sampling strategies for endophyte testing vary depending upon the objective of the research. Plant breeders are likely to be most concerned about individual plants during initial screening and selection. Selection of a single mature tiller can be subsampled by excising a 4-mm cross section of the tiller base for immunoblot analysis of endophyte, and an additional 4-mm cross section for qualitative alkaloid analysis. This strategy permits a plant breeder to examine thousands of plants per day once the samples have been collected. Once populations of plants have been selected and advanced to small plot trials, immunoblotting for endophyte and ELISA analysis can be performed to examine the stability of the endophyte and alkaloid traits among breeding populations tested across locations (Hill et al., 2002). Small plot sampling strategies vary depending upon the experiment, but for breeding purposes it is more important to characterize the plot for infection and alkaloid status and relatively simple sampling procedures can be used. Breeding populations were planted at two locations and replicated 6 times, only 8 tillers per treatment were collected in each replication (48 total per location) in each year of the experiment to characterize the endophyte status of the treatments. The same study had alkaloids analyzed by ELISA from the harvested forage for each clipping date over a 3-year period. This sampling strategy was sufficient to characterize the endophyte and alkaloid status and the populations. When populations are advanced to grazing studies, a similar system can be used to analyze for endophyte and alkaloids in pastures, with sampling conducted on a monthly basis to characterize the trends in alkaloids.
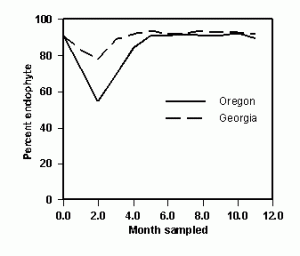
Regardless of the experiment, endophyte sampling must be performed in months when the endophyte fully expresses itself in the plant. An experiment was conducted to compare monthly infection rates of tall fescue pastures or seed fields located in Georgia and Oregon, respectively. Infection rates were stable at both locations when sampled from June through January, but the months of February through April had reduced endophyte levels, especially in Oregon (Figure 1). The two sampling sites represent geophysical extremes within the US, and extrapolation of the data to other locations is not feasible. Hence, similar studies need to be performed throughout the US to illuminate the limitations for endophyte sampling, and until then, sampling should be limited to summer and fall months. Once the new non-toxic cultivar has been developed there are regulatory and quality assurance issues that must be addressed to satisfy concerns over endophyte viability and toxicity of the final product.
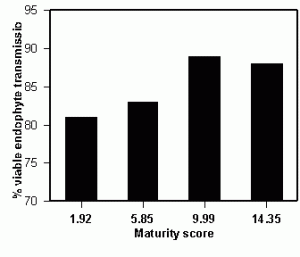
Figure 2. Endophyte infection frequencies of seeding plants when seed were harvested at different stages of maturity.
Normally, seed traits (germination, viability, etc.) are based upon a minimal analysis of 400 seeds (AOSA, 2001). The tedium, monotony, and time constraints of microscopic analysis have limited the extensiveness of testing for endophyte in many regulatory labs. In fact, there are some regulatory agencies that have adopted a testing protocol that is based upon probabilities of finding endophyte when testing seed in batches of ten. That is, if ten seed are tested and there is no endophyte, another ten are tested, and so on until the probability of finding endophyte meets a statistical criteria. In some cases, seed lots are tested based upon analysis of 30 seeds. This method of sample size reduction, while convenient for the testing laboratory, limits the rigors of the test, making it prone to error. In the case of MaxQ, the minimal criteria established by AOSA are exceeded and internal standards for quality assurance designed to provide the end-user with a known quality product.
It is well known the endophyte dies preferentially to the seed when in storage. The mechanisms of endophyte viability and longevity are poorly understood, and therefore, provide an opportunity for other academic disciplines to play a supporting role for plant breeders and the seed industry. One concern is how endophytes prepare for the final dessication event of seed maturity, and whether truncating this process by early harvest might influence the viability of endophtyes during storage. Tall fescue is a semi-determinant flowering plant, thus, seed within a panicle vary in maturity and panicles within and among plants vary in maturity as well. Hence, a staging system of evaluating seed and panicle color was developed and seed harvested at varying stages of their development. The seed ranged from almost entirely green (maturity score = 1.92) to nearly all straw colored (maturity score 14.35). Seed were sampled from four fields (replications) and the seed chilled at 4oC for 0, 3, 5, or 7 days to break dormancy. Fifty seed were tested from each chilling treatment for each rep, for a total of 800 seedling evaluations per data point. Seeds harvested at the earlier maturity stages had lower viable endophyte levels than those from later maturity levels with a probability level of 0.04. These types of studies are descriptive in nature and do not provide the basic information as to mechanisms of viability. However, they are necessary to conduct preliminary tests to provide insight as to hypotheses of the nature of the seed/endophyte interaction. The point to be taken is that understanding the biology of the system will require more extensive endophyte testing and it will likely be a complicated scientific effort to understand the biology of the association between endophyte and seed host. Having a rapid method of analysis will be vital for the next generation of scientific technology utilizing endophtyes for forage improvement.
References
Association of Official Seed Analysts. 2001. Rules fo r testing seed. AOSA, Las Cruces, NM.
Adcock, R.A., N.S. Hill, J.H. Bouton, H.R. Boerma, and G.O. Ware. 1997. Symbiont regulation and reducing ergot alkaloid concentration by breeding endophyte-infected tall fescue. Journal of Chemical Ecology 23:691-704..
Agee, C.S. and N.S. Hill. 1994. Environmental and plant genotypic effects on ergovaline content in tall fescue. Crop Science 34:221-226.
Goding, J.W. 1986. Monoclonal antibodies: Principles and practice. 2nd Edition. Academic Press. San Diego, CA.
Gwinn, K.D., M.H. Collins-Shepard, and B.B. Reddick. 1991. Tissue print-immunoblot, an accurate method for the detection of Acremonium coenophialum in tall fescue. Phytopathology 81:747-748.
Hiatt, E.E., III, N.S. Hill, J.A. Bouton, and C.W. Mims. 1997. Monoclonal antibodies for detection of Neotyphodium coenophialum. Crop Science 37:1265-1269.
Hiatt, E.E., III, and N.S. Hill. 1999. Tall fescue endophyte detection: Tissue immunoblot test kit compared with microscopic analysis. Crop Science 39:796-799.
Hill, N.S., G.E. Rottinghaus, C.S. Agee, and L.M. Schultz. 1993. Simplified sample preparation for HPLC analysis of ergovaline in tall fescue. Crop Science 33:331-333.
Hill, N.S., F.N. Thompson, D.L. Dawe, and J.A. Stuedemann. 1994. Antibody binding of circulating ergopeptine alkaloids in cattle grazing tall fescue. American Journal of Veterinary Research 55:419-424.
Hill, N.S., and C.S. Agee. 1994. Detection of ergopeptine alkaloids in endophyte-infected tall fescue populations by immunoassay. Crop Science 34:530-534.
Hill, N.S., E.E. Hiatt, J.P. DeBattista, M.C. Costa, C.H. Griffiths, J. Klap, D. Thorogood, and J. Reaves. 2002. Seed testing for endophytes by microscopic and immunoblot procedures. Seed Science and Techology (In Press)
Hill, N.S., J.H. Bouton, F.N. Thompson, L. Hawkins, C.S. Hoveland, and M.A. McCann. 2002. Performance of tall fescue germplasms bred for high- and low-ergot alkaloids. Crop Science (In Press).
Reddick, B.B. and M.H. Collins. 1988. An imporved method for detection of Acremonium coenophialum in tall fescue plants. Phyotpathology. 78:418-420.
Roitt, I.M. 1994. Essential immunology. 8th Edition. Blackwell Scientific Publications. Cambridge, MA.
Roylance, J.T., N.S. Hill, and C.S. Agee. 1994. Ergovaline and peramine production in endophyte-infected tall fescue: Independent regulation and effects of plant and endophyte genotype. Journal of Chemical Ecology 20:2171-2183.
Welty, R.E. M.D. Azevedo, and K.L. Cook. 1986a. Detecting viable Acremonium endophytes in leaf sheaths and meristems of tall fescue and perennial ryegrass. Plant Dis. 431-435.
Welty, R.E., G.M Milbrath, P. Faulkenberry, M.D. Azevedo, L. Meek, and K. Hall. 1986. Endophytic detection in tall fescue seed by staining and ELISA. Seed Sci. Technol. 14:105-116.