Looking Beyond Dewormers
This edition of Reid’s Ram-blings was composed by Jake Thorne, Texas A&M AgriLife Extension Associate. Jake has been a contributing editor to this column over the past year and is a valued asset to the sheep and goat Extension team at the San Angelo center. Enjoy!
The destructive power of microscopic bio-agents may not have been on your mind two months ago. We can now throw that blissful lack of worry right out the window. COVID-19 has brought the world to a screeching halt, but you are undoubtedly seeking a new topic for your daily dose of reading, so here’s something that may, or may not, put your mind at ease. Your sheep and goats have parasites, specifically haemonchus contortus, and these worms are killing your animals. See, don’t you feel better already?
In all seriousness, internal parasites are not a new theme or topic if you’re a long-time reader of this column. However, they are perhaps the single greatest issue we face in the sheep and goat industry here in Texas and are worth discussing every chance we get. If you are looking for drenching protocols or how to perform fecal egg counts, we have some great videos on these topics on our Facebook page and a sister YouTube channel. I suggest you check it out if you haven’t already done so. With that said, I want to dive a little deeper into a different aspect of parasite management; identifying animals that are resistant, and what that word really means.
Some sheep and goats are able to withstand parasitic infections from the barber pole worm (H. Contortus) much better than others. The barber pole worm resides in the abomasum of ruminant livestock and uses a spear-like tail to puncture the lining of the stomach and feed on the blood of the animal. In the meantime, female worms shed around 5,000 eggs per day. Barbaric and prolific…an effective combination for survival! While mechanisms for host resistance to barber pole worms are complex and still being discovered, research has consistently linked more resistant sheep with higher levels of Th2 helper cell activity, aka a stronger immune response. Immune cells that defend the host against parasites (eosinophils), quicker tissue repair, mucosa secretion in the gastrointestinal tract, and IgA antibody production are all results of increased Th2 activity.
Considering worm resistance to drenches is rampant and that approval of new anthelmintics is not on the horizon, selecting for animals that are genetically capable of combating parasites is crucial. I may be going out on a limb, but in my opinion this may actually be the single most important selection criteria you should have. With that said, one of the greatest mistakes producers make is believing certain animals who are not showing outward signs of parasites are more resistant when in reality they have just not experienced the same “stress” as others in the flock. For example, let’s say we have two sheep, Shrek and Dolly. We have performed a fecal egg count (FEC) on both (a FEC is a way of quantifying the eggs that are shed from the animal in the fecal matter thus allowing us to estimate the worm load in the animal). Shrek has a FEC of 500 eggs per gram (epg) and Dolly has a FEC of 3,000 epg. Seems like a no brainer as to which is more resistant, right? However, what if Shrek did not raise a lamb this year and Dolly currently has a set of twins? These two animals are not able to be accurately compared because the environmental effect (everything that affects an animal other than its genetics) is skewed. We know something called the periparturient rise exists, which is a situation where at the time of parturition the immune system of the dam becomes suppressed and parasites typically flourish, potentially confounding the comparison described here.
Therefore, to identify animals that are more resistant to parasites, we need to level the environmental playing field. The preferred way to do this is to identify a contemporary group of lambs or kids, manage them similarly, and then before they enter into production (lambing or kidding) collect fecal samples and have these analyzed. Common times to collect are at weaning, 60-90 days post weaning or when the animals are a year of age. Instead of having preset dates however, the correct time to collect is when the animals have had some exposure to parasites (they aren’t naïve) and are carrying a moderate load.
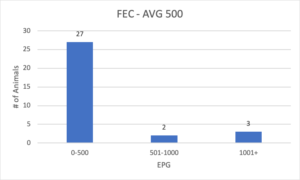
A moderate load can be defined when the average of the contemporary group is between 500 and 1,000 epg. One thing to keep in mind is that the correlation between levels of genetic resistance and actual fecal egg counts are not linear, i.e. you likely won’t find a steady rise in the egg counts of this group. More likely, most animals will be at or below the average and a few will be as much as five to 10 times the average! For example, in 2019 at the TAMU Research Station in San Angelo we analyzed FECs on 32 six-month-old Rambouillet sheep. The egg counts ranged from 0 to 4,350 epg, and results are depicted in the bar graph below:
From the analysis we learn that 27 of the 32 animals in the group are at or below the contemporary group average (500 eggs per gram). With that said, the top 5 head (~15%) with the highest egg counts are shedding 72% of the eggs for the entire group. Let’s say each of these animals is producing 1 kilogram of feces a day, we can multiply their egg count by 1,000 to get the number of potential parasites they are shedding on pasture each day. The five sheep with the highest egg counts in this group were dropping a combined 10.6 million eggs per day! The take-away here isn’t that 500 epg is a magical threshold, more-so that you will find the majority of your flock’s parasites come from a small percentage of your animals. These reservoir animals are the ones we want to identify and select against. It’s important to keep in mind that though they may not have visible signs of parasites (a “resilient” animal wouldn’t), they are still a major contributor to the amount of parasites out in the pasture and being consumed by the rest of the flock.
Raw fecal egg counts are clearly useful for identifying some animals with lower resistance, but the best way currently available to sheep and goat producers to determine genetic resistance to parasites is through using estimated breeding values (EBVs) from the National Sheep Improvement Program (NSIP). New this year, NSIP will be offering fecal egg count EBVS for all breeds of sheep, which can be a powerful tool for Texas producers who consistently have major issues with the barber pole worm. EBVs are calculated not only on an individual animal’s performance, but also on the performance of all of its relatives (offspring included). Ultimately, combating parasites is a complex problem and requires a multifaceted approach to solve it. Identifying resistant animals and keeping those as replacements is only one piece of the puzzle.
Reflecting on the year 2020 so far, we have experienced a situation unlike any my generation has experienced. While the time working from home has its inconveniences, I am certainly thankful for the added family-time it has provided, as well as extra opportunities to contemplate my own sheep operation’s management plan. There is nothing like spending time with your kids, out in the barn, gathered around sheep, collecting poop samples. Welcome to your anatomy and physiology lesson kids!
To provide feedback on this article or request topics for future articles, contact me at reid.redden@ag.tamu.edu or 325-657-7324. For general questions about sheep and goats, contact your local Texas A&M AgriLife Extension Service county office. If they can’t answer your question, they have access to someone who can.